Deciphering the Allosteric Effect of Antagonist Vismodegib on Smoothened Receptor Deactivation Using Metadynamics Simulation
- PMID: 31214579
- PMCID: PMC6558189
- DOI: 10.3389/fchem.2019.00406
Deciphering the Allosteric Effect of Antagonist Vismodegib on Smoothened Receptor Deactivation Using Metadynamics Simulation
Abstract
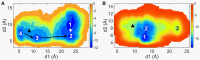
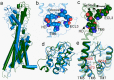
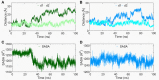
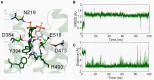
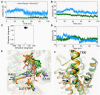
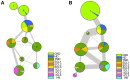
The smoothened receptor (Smo) plays a key role in Hedgehog (Hh) signaling pathway and it has been regarded as an efficacious therapeutic target for basal cell carcinoma (BCC) and medulloblastoma (MB). Nevertheless, the resistance mutation and active mutants of Smo have put forward the requirement of finding more effective inhibitors. Herein, we performed metadynamics simulations on Smo bound with vismodegib (Smo-Vismod) and with cholesterol (Smo-CLR), respectively, to explore the inhibition mechanism of vismodegib. The simulation results indicated that vismodegib-induced shifts of TM5, TM6, and TM7, which permitted the extracellular extension of TM6 and extracellular loop3 (ECL3) to enter the extracellular cysteine-rich domain (CRD) groove. Therefore, an open CRD groove that has not been noticed previously was observed in Smo-Vismod complex. As a consequence, the occupied CRD groove prevents the binding of cholesterol. In addition, the HD and ECLs play crucial roles in the interaction of CRD and TMD. These results reveal that TM5, TM6, and TM7 play important roles in allosteric inhibition the activation of Smo and disrupting cholesterol binding by vismodegib binding. Our results are expected to contribute to understanding the allosteric inhibition mechanism of Smo by vismodegib. Moreover, the detailed conformational changes contribute to the development of novel Smo inhibitors against resistance mutation and active mutants of Smo.
Keywords: allosteric inhibition mechanism; cholesterol; metadynamics simulation; smoothened receptor; vismodegib.
Figures
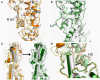

Similar articles
-
Structural dynamics of Smoothened (SMO) in the ciliary membrane and its interaction with membrane lipids.Biochim Biophys Acta Biomembr. 2022 Aug 1;1864(8):183946. doi: 10.1016/j.bbamem.2022.183946. Epub 2022 Apr 25. Biochim Biophys Acta Biomembr. 2022. PMID: 35483421
-
Molecular basis of drug resistance in smoothened receptor: An in silico study of protein resistivity and specificity.Proteins. 2020 Mar;88(3):514-526. doi: 10.1002/prot.25830. Epub 2019 Oct 16. Proteins. 2020. PMID: 31589795
-
A sterol analog inhibits hedgehog pathway by blocking cholesterylation of smoothened.Cell Chem Biol. 2024 Jul 18;31(7):1264-1276.e7. doi: 10.1016/j.chembiol.2024.02.002. Epub 2024 Mar 4. Cell Chem Biol. 2024. PMID: 38442710
-
Targeting the Oncoprotein Smoothened by Small Molecules: Focus on Novel Acylguanidine Derivatives as Potent Smoothened Inhibitors.Cells. 2018 Dec 14;7(12):272. doi: 10.3390/cells7120272. Cells. 2018. PMID: 30558232 Free PMC article. Review.
-
Overcoming the emerging drug resistance of smoothened: an overview of small-molecule SMO antagonists with antiresistance activity.Future Med Chem. 2018 Dec;10(24):2855-2875. doi: 10.4155/fmc-2018-0200. Epub 2018 Dec 17. Future Med Chem. 2018. PMID: 30557039 Review.
References
-
- Bai Q., Shen Y., Jin N., Liu H., Yao X. (2014). Molecular modeling study on the dynamical structural features of human smoothened receptor and binding mechanism of antagonist LY2940680 by metadynamics simulation and free energy calculation. Biochim. Biophys. Acta 1840, 2128–2138. 10.1016/j.bbagen.2014.03.010 - DOI - PubMed
-
- Bender M. H., Hipskind P. A., Capen A. R., Cockman M., Credille K. M., Gao H., et al. (2011). Identification and characterization of a novel smoothened antagonist for the treatment of cancer with deregulated hedgehog signaling. Cancer Res. 71, 2819–2819. 10.1158/1538-7445.AM2011-2819 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

