The AAA-ATPase MIDASIN 1 Functions in Ribosome Biogenesis and Is Essential for Embryo and Root Development
- PMID: 30755475
- PMCID: PMC6501072
- DOI: 10.1104/pp.18.01225
The AAA-ATPase MIDASIN 1 Functions in Ribosome Biogenesis and Is Essential for Embryo and Root Development
Abstract
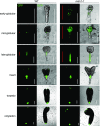
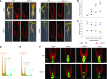
Ribosome biogenesis is an orchestrated process that relies on many assembly factors. The AAA-ATPase Midasin 1 (Mdn1) functions as a ribosome assembly factor in yeast (Saccharomyces cerevisiae), but the roles of MDN1 in Arabidopsis (Arabidopsis thaliana) are poorly understood. Here, we showed that the Arabidopsis null mutant of MDN1 is embryo-lethal. Using the weak mutant mdn1-1, which maintains viability, we found that MDN1 is critical for the regular pattern of auxin maxima in the globular embryo and functions in root meristem maintenance. By detecting the subcellular distribution of ribosome proteins, we noted that mdn1-1 impairs nuclear export of the pre-60S ribosomal particle. The processing of ribosomal precusor RNAs, including 35S, 27SB, and 20S, is also affected in this mutant. MDN1 physically interacts with PESCADILLO2 (PES2), an essential assembly factor of the 60S ribosome, and the observed mislocalization of PES2 in mdn1-1 further implied that MDN1 plays an indispensable role in 60S ribosome biogenesis. Therefore, the observed hypersensitivity of mdn1-1 to a eukaryotic translation inhibitor and high-sugar conditions might be associated with the defect in ribosome biogenesis. Overall, this work establishes a role of Arabidopsis MDN1 in ribosome biogenesis, which agrees with its roles in embryogenesis and root development.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures






Similar articles
-
Arabidopsis NOTCHLESS plays an important role in root and embryo development.Plant Signal Behav. 2023 Dec 31;18(1):2245616. doi: 10.1080/15592324.2023.2245616. Plant Signal Behav. 2023. PMID: 37573563 Free PMC article.
-
Coordination between MIDASIN 1-mediated ribosome biogenesis and auxin modulates plant development.J Exp Bot. 2021 Mar 29;72(7):2501-2513. doi: 10.1093/jxb/erab025. J Exp Bot. 2021. PMID: 33476386
-
Mutation of an Essential 60S Ribosome Assembly Factor MIDASIN 1 Induces Early Flowering in Arabidopsis.Int J Mol Sci. 2022 Jun 10;23(12):6509. doi: 10.3390/ijms23126509. Int J Mol Sci. 2022. PMID: 35742952 Free PMC article.
-
ATAD3 Proteins: Unique Mitochondrial Proteins Essential for Life in Diverse Eukaryotic Lineages.Plant Cell Physiol. 2024 May 14;65(4):493-502. doi: 10.1093/pcp/pcad122. Plant Cell Physiol. 2024. PMID: 37859594 Review.
-
Small signaling peptides in Arabidopsis development: how cells communicate over a short distance.Plant Cell. 2012 Aug;24(8):3198-217. doi: 10.1105/tpc.112.099010. Epub 2012 Aug 28. Plant Cell. 2012. PMID: 22932676 Free PMC article. Review.
Cited by
-
Arabidopsis NOTCHLESS plays an important role in root and embryo development.Plant Signal Behav. 2023 Dec 31;18(1):2245616. doi: 10.1080/15592324.2023.2245616. Plant Signal Behav. 2023. PMID: 37573563 Free PMC article.
-
The uS10c-BPG2 module mediates ribosomal RNA processing in chloroplast nucleoids.Nucleic Acids Res. 2024 Jul 22;52(13):7893-7909. doi: 10.1093/nar/gkae339. Nucleic Acids Res. 2024. PMID: 38686791 Free PMC article.
-
Maize Shrek1 encodes a WD40 protein that regulates pre-rRNA processing in ribosome biogenesis.Plant Cell. 2022 Sep 27;34(10):4028-4044. doi: 10.1093/plcell/koac216. Plant Cell. 2022. PMID: 35867001 Free PMC article.
-
Exploring the Potential Role of Ribosomal Proteins to Enhance Potato Resilience in the Face of Changing Climatic Conditions.Genes (Basel). 2023 Jul 18;14(7):1463. doi: 10.3390/genes14071463. Genes (Basel). 2023. PMID: 37510367 Free PMC article.
-
NAC1 Maintains Root Meristem Activity by Repressing the Transcription of E2Fa in Arabidopsis.Int J Mol Sci. 2022 Oct 14;23(20):12258. doi: 10.3390/ijms232012258. Int J Mol Sci. 2022. PMID: 36293114 Free PMC article.
References
-
- Abbasi N, Kim HB, Park NI, Kim HS, Kim YK, Park YI, Choi SB (2010) APUM23, a nucleolar Puf domain protein, is involved in pre-ribosomal RNA processing and normal growth patterning in Arabidopsis. Plant J 64: 960–976 - PubMed
-
- Barrio-Garcia C, Thoms M, Flemming D, Kater L, Berninghausen O, Bassler J, Beckmann R, Hurt E (2016) Architecture of the Rix1-Rea1 checkpoint machinery during pre-60S-ribosome remodeling. Nat Struct Mol Biol 23: 37–44 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

